This Gene therapy used a lentiviral vector to introduce a normal copy of the Factor VIII gene into autologous hematopoietic stem cells (HSCs) to treat Haemophilia A (refer to box).
- Lentiviral vectors are a type of viral vector that can be used to transfer genetic material.
- HSCs are multipotent primitive cells that can develop into all types of blood cells.
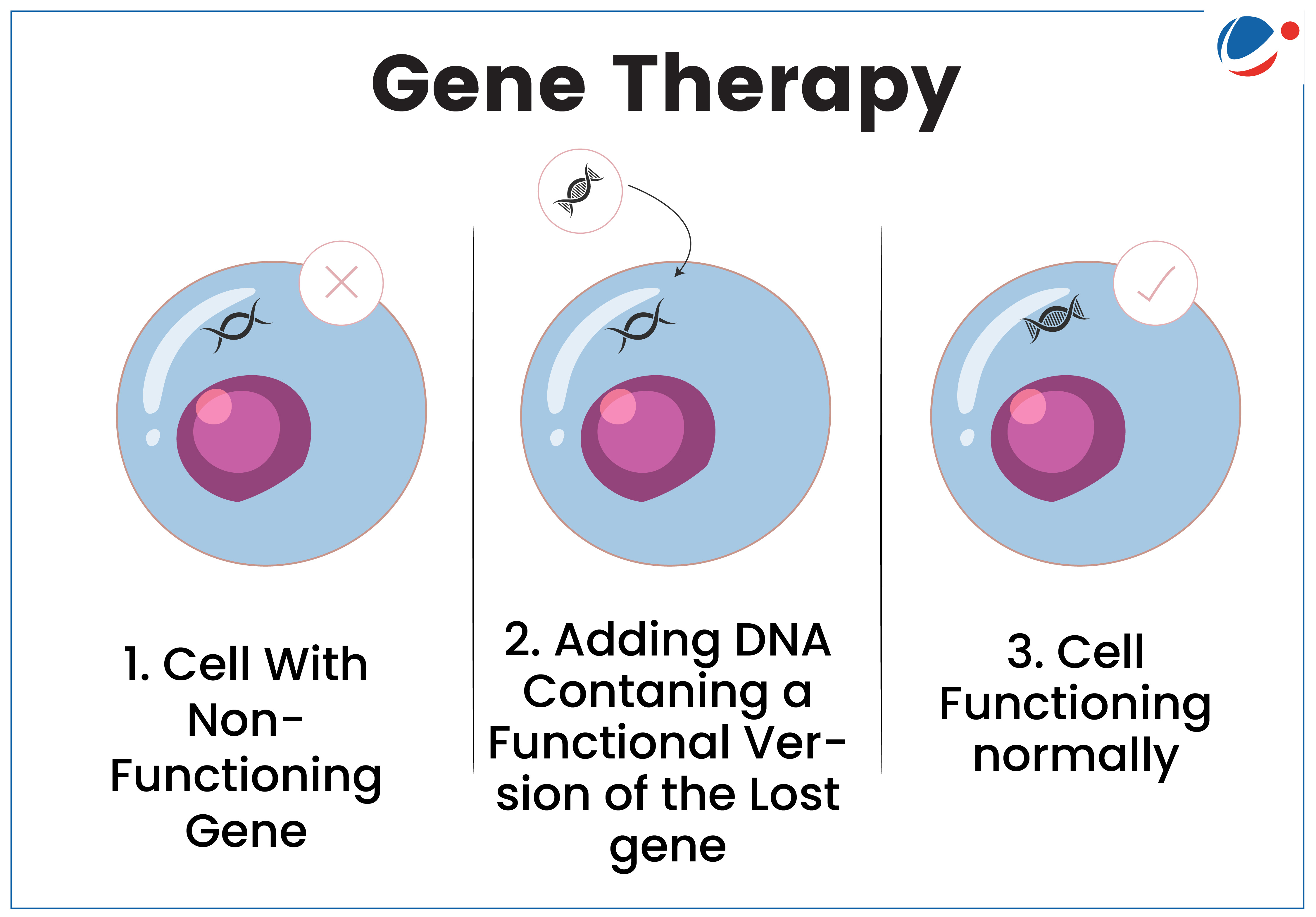
About Gene therapy
- Gene therapy is a technique that uses a gene(s) to treat, prevent or cure a disease or medical disorder.
- It works by replacing faulty genes, deactivating harmful ones, or introducing new genes to improve or restore health.
- It uses products/methods such as Plasmid DNA (Genetically engineered Circular DNA molecules), Human gene editing technology, etc.
- Types of Gene Therapy
- Germline gene therapy: In it, the Germline Cell (egg or sperm) are modified by the introduction of functional genes.
- Somatic cell gene therapy: In this, therapeutic gene are transferred to a patient’s somatic cells (cells other than germline cells).
- Application: both inherited genetic diseases (e.g., sickle cell disease) and acquired disorders (e.g., leukemia) could be treated.
About Haemophilia
|



