Blindsight is an experimental vision-restoring implant which aims to assist individuals who have lost their sight or were born blind (Initially offering low-resolution vision).
- Breakthrough device designation is intended to accelerate development of devices that offer promising new treatments or diagnostic capabilities for life-threatening conditions.
- Neuralink, founded by Elon Musk in 2016, is a company working on Brain Computer Interfaces (BCIs) with a focus on healthcare applications.
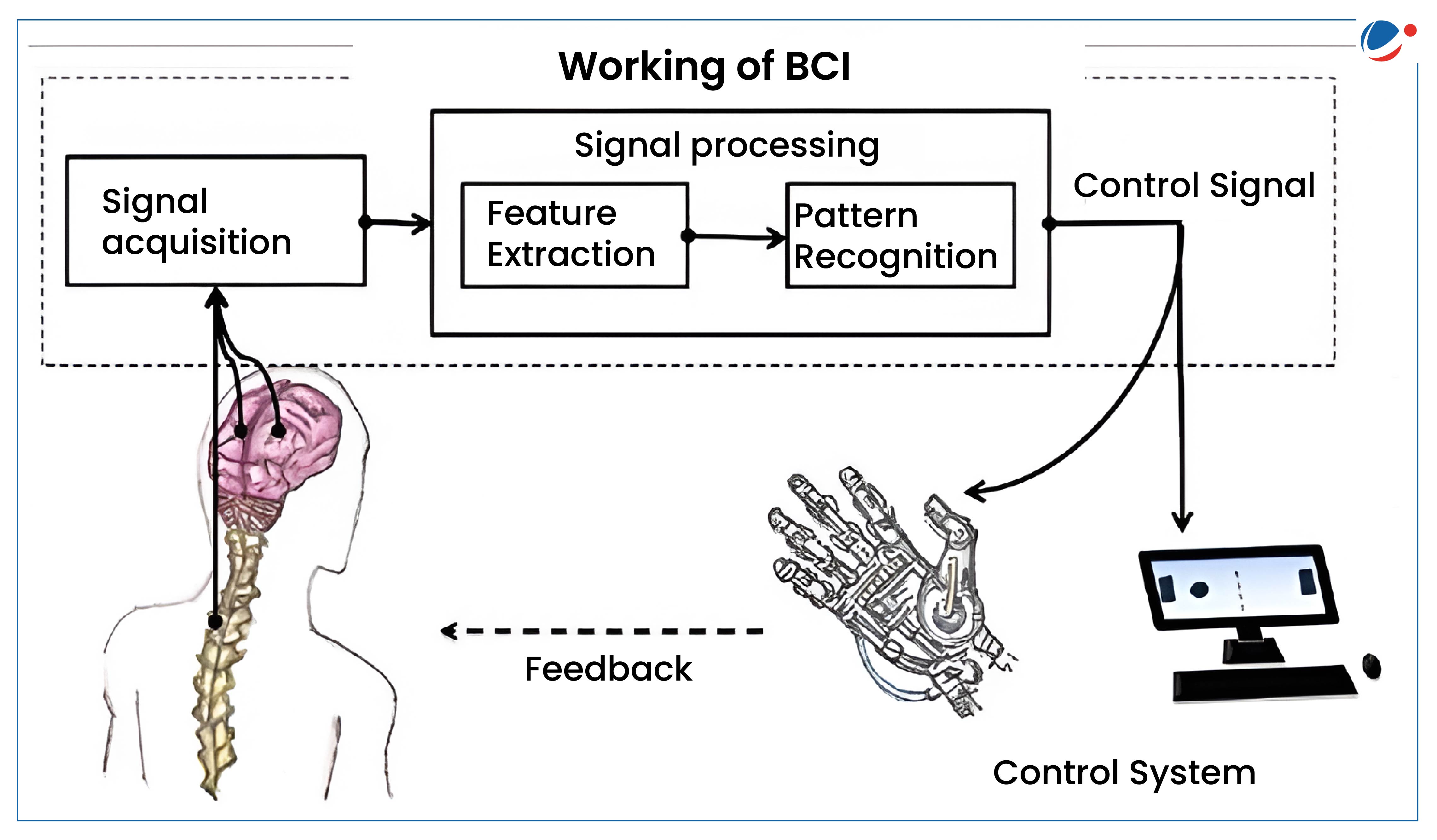
About BCIs:
- It allows users to communicate or control external devices using brain signals without any physical movement.
- Types of BCIs:
- Invasive: Chips/Sensors are placed directly into the cortex E.g. Neuralink’s Implant.
- Non-Invasive: Sensors are placed on scalp
- Partially invasive: implanted inside skull but rest outside brain.
Applications of BCIs:
- Medical: Help restore lost functions e.g. controlling prosthetic limbs or aiding patients with neurological disorders like paralysis
- Mental wellness: Real-time feedback on users’ mental well-being and facilitates effective mental health practices.
- Defense: Improved battlespace awareness, enhanced management of autonomous systems etc.
- Gaming and entertainment: Immersive gaming experiences through uni- or bi-directional neural communication.
Issues with BCIs:
- Cybersecurity risks: E.g. Brain tapping(intercepting signals transmitted from brain)
- Ethical Concerns: concerns regarding informed consent
- Safety Issues: E.g. tissue damage, seizures, cognitive impairment etc.




