Why in the news?
Central Drugs Standard Control Organisation (CDSCO) directed manufacturers of 49 medicines to recall their products after samples were found to be "not of standard quality".
More on the News
- Drug samples that failed quality tests included Metronidazole tablets (for treating infections); Oxytocin injection; Metformin hydrochloride (helps control amount of blood glucose; Diclofenac sodium tablets (painkiller) etc.
- Every month, drugs samples are picked from sales/distribution point, analyzed and list of Spurious Drugs and Not of Standard Quality (NSQ) Drugs are displayed on CDSCO portal.
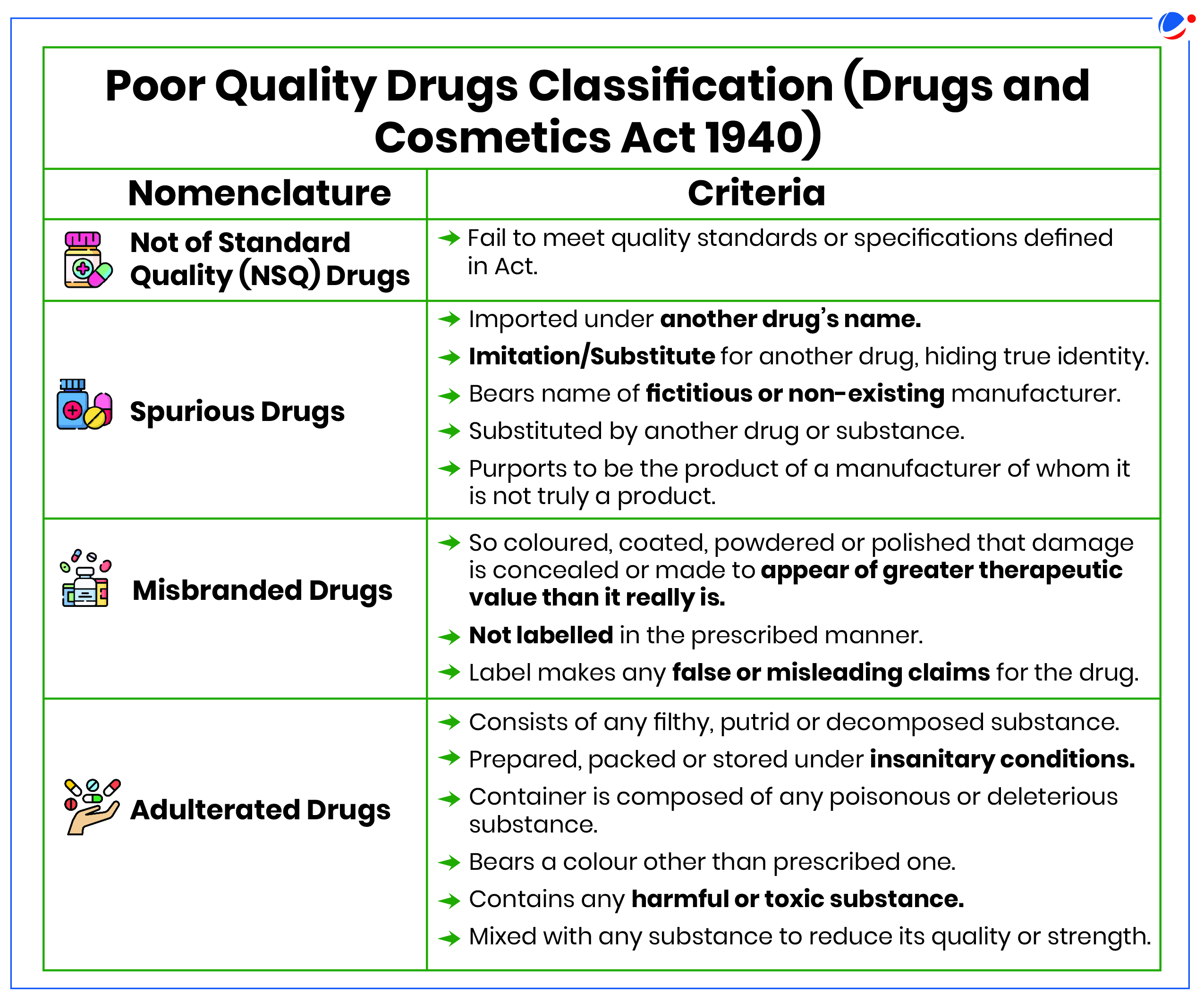
Regulation of drugs in India
- CDSCO: Central Drugs Standard Control Organisation (CDSCO), under Ministry of Health and Family Welfare, is primary regulatory body for pharmaceutical sector.
- It regulates quality, safety and efficacy of Drugs, Medical Device and Cosmetics in the country under the provisions of Drugs & Cosmetics Act, 1940 and Drugs and Cosmetics Rules, 1945.
- Drugs and Cosmetics Act (DCA), 1940: It along with Drugs and Cosmetics Rules, 1945, regulates import, manufacturing, sale and distribution of drugs in India.
- Regulatory control is exercised through a system of licensing and inspection by the State Licensing Authorities.
- State Drug Regulatory Authorities (SDRAs): Responsible for licensing of manufacturing establishments, surveillance over sale of spurious drugs, instituting legal prosecution and monitoring of objectionable advertisements.
- Statutory Bodies: DCA 1940 provides for establishment of:
- Drugs Technical Advisory Board (DTAB): Guides and advises central government on technical issues arising out of implementation of regulation.
- Drugs Consultative Committee (DCC): An advisory committee to advise central government, state governments and DTAB on any matter tending to secure uniformity in administration of DCA.
- Central Drugs Laboratory (CDL): National statutory laboratory for quality control of Drug and Cosmetics.
Issues with drug quality in India
- Weak Enforcement: Division of regulatory responsibilities between the centre and states, without any single agency has led to fragmentation and undermined effectiveness.
- Challenges to State-Level Authorities (SLAs): SLAs face challenges like ill-equipped testing labs, paucity of drug inspectors, poor understanding of rules, patchy surveillance and lack of legal expertise to take action against violators.
- Non-Compliance with Standards: In 2023, just about 2,000 out of 10,500 manufacturing units were found to be compliant with WHO- Good Manufacturing Practices (GMP) standards.
- Bulk of API is imported from China, Taiwan, among other countries, which requires effective quality monitoring.
- Financial Disbursement: SDRAs and CDSCO are financially reliant on government funding and have to go through a complicated system of approvals for financial disbursement.
- Information Asymmetry: This is due to non-mention of time frame for completion of regulation stages, no centralised record keeping, absence of national database of manufacturers and non-uniformity in implementation of law.
- Limited reach of Pharmacovigilance: Limited outreach of Pharmacovigilance Programme of India among patients as well as healthcare professionals, with little or no information about measures taken after adverse drug reports.
- Data Integrity: There are data integrity problems found in Indian manufacturers of bioavailability (to establish the in vivo performance of a new drug product) and bioequivalence (to compare the performance of the original formulation to a to-be marketed drug product) studies.
Measures taken to ensure quality of Drugs
- Strengthening of States' Drug Regulatory System (SSDRS): It is a Centrally Sponsored Scheme which envisages to strengthen the laboratory infrastructure and up-gradation of existing State Drug Controller offices in States.
- Amendment to DCA 1940: Drugs & Cosmetics (Amendment) Act 2008 provides stringent penalties for manufacture of spurious and adulterated drugs and made certain offences cognizable and non-bailable.
- Amendments to Drugs and Cosmetics Rules, 1945: Making inspection of manufacturing establishment mandatory by Central and State Drug Inspectors before the grant of manufacturing license.
- Revised the schedule M to the rules to integrate WHO- GMP standards.
- Revamping Pharmaceuticals Technology Upgradation Assistance Scheme (PTUAS): Government has expanded the eligibility beyond MSMEs to include any pharmaceutical manufacturing unit with a turnover of below Rs 500 crore that requires technology and quality upgradation.
- Special Courts: States/UTs have set up special Courts for trial of offences under DCA for speedy disposal.
Way Forward
- Uniformity: Ensure uniform drug regulatory standards nationwide by greater centralisation of powers in CDSCO and empowering SDRAs to partner effectively with CDSCO through an expanded role of DCC.
- Strengthen Regulation: Need to priorities and invest in regulatory resources on aspects such as granting manufacturing licenses, inspections, sampling and testing (overall drug quality).
- Financing: Financial autonomy in revenue generation and disbursement for efficient functioning of drug regulatory authorities will address delays arising from complicated and lengthy approval systems for financial disbursement.
- Leveraging digital technologies to significantly enhance patient safety monitoring and pharmacovigilance.



